Technology Power
Here you can find information about metal, enjoy it :)
martes, 16 de diciembre de 2014
lunes, 15 de diciembre de 2014
Video
This is the greatest and the coolest video that I could find in the hole youtube , because it´s the funnier.
It explains a lot about metals , how they are form and which are the components of them . In my opinion this video will help you to know the periodic table by hard and all that staff, I hope you enjoy it :)
lunes, 8 de diciembre de 2014
Metals
Metals
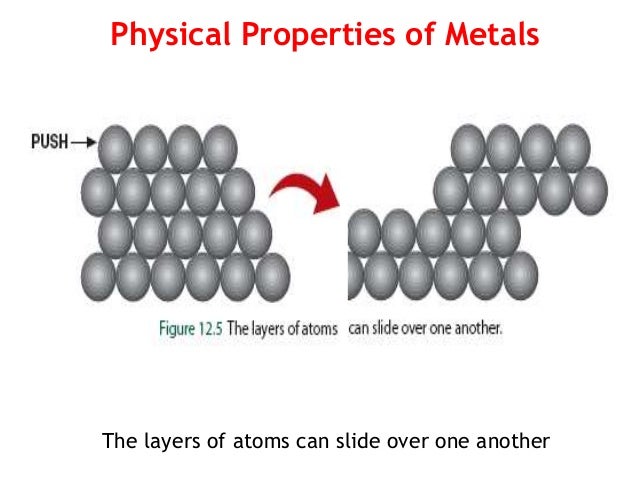
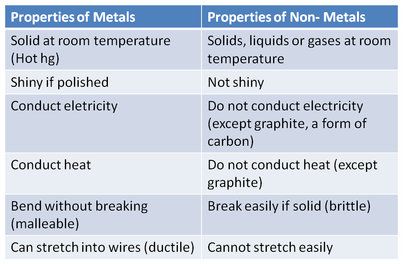
- Metals are materials wich are normally : hard, opaque, shiny, and has good electrical and thermal conductivity. They are malleable that means, that they can be pressed with other shapes whitout breaking althought this is joint to fusible in one way because by fusibling something it´s melting.Also we are having the ductile wich means that over 91 of the 118 metals are travelling trought a wire
Examples :

Fusible
Malleable
Properties
The metals has the chemical , physical electrical and mechanical :
Chemical properties: Metals are usually inclined to form cations with oxygen in the air and forms oxides over various timescales (iron rusts over years, while potassium burns in seconds). Examples:
- 4 Na + O2 → 2 Na2O (sodium oxide)
- 2 Ca + O2 → 2 CaO (calcium oxide)
- 4 Al + 3 O2 → 2 Al2O3 (aluminium oxide
- The oxides of metals are generally basic, as opposed to those of non-metals, which are acidic. Blatant exceptions are largely oxides with very high oxidation states such as CrO3, Mn2O7, and OsO4, which have strictly acidic reactions.
Physical properties: Metals in general have high electrical conductivity, high thermal conductivity, and high density. Typically they are malleable and ductile, deforming under stress without cleaving. In terms of optical properties, metals are shiny and lustrous. Sheets of metal beyond a few micrometres in thickness appear opaque, but gold leaf transmits green light.

Electrical propierties : The electrical and thermal conductivities of metals originate from the fact that their outer electrons are delocalized. This situation can be visualized by seeing the atomic structure of a metal as a collection of atoms embedded in a sea of highly mobile electrons
it is necessary to take into account the positive potential caused by the specific arrangement of the ion cores
mechanical properties : mechanical properties of metals include ductility;their capacity for plastic deformation. Reversible elastic deformation in metals can be described by Hooke's Law for restoring forces, where the stress is linearly proportional to the strain. Forces larger than the elastic limit, or heat, may cause a permanent (irreversible) deformation of the object, known as plastic deformation or plasticity. This irreversible change in atomic arrangement may occur as a result of:
* The action of an applied force (or work). An applied force may be tensile (pulling) force, compressive (pushing) force, shear, bending or torsion (twisting) forces.
* A change in temperature (heat). A temperature change may affect the mobility of the structural defects such as grain boundaries, point vacancies, line and screw dislocations, stacking faults and twins in both crystalline and non-crystalline solids. The movement or displacement of such mobile defects is thermally activated, and thus limited by the rate of atomic diffusion.
Suscribirse a:
Entradas (Atom)